UK Responsible Person (UKRP) Services for Cosmetics Compliance
Expert Guidance for Effortless Cosmetics Importing into the UK
We understand the complexities of importing cosmetics into the UK Market
Why Appoint a UK Responsible Person for Your Cosmetic Products?
Since the UK’s departure from the European Union on January 31, 2020, the requirement for a UK Responsible Person (UKRP) has become essential for all companies wishing to sell cosmetic products in Great Britain. Under the “Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019, Schedule 34,” commonly known as the “UK Cosmetics Regulation,” companies were required to comply by December 31, 2020, at the end of the transition period.
From January 31, 2020, onwards, any business aiming to market cosmetic products in the UK must appoint a Responsible Person (RP) located within the UK for each product. The RP—sometimes referred to as the UK Legal Representative or UK Legal Representation—ensures ongoing compliance with UK cosmetics regulations and safeguards human health.
A Responsible Person is either a legal or natural entity based in the UK, such as the manufacturer (if located in the UK), an importer, a distributor, or a third-party representative who has been designated by a formal mandate to ensure product compliance in the UK market.
Our Comprehensive UKRP Services for Cosmetics
As your appointed Responsible Person in the UK, Eomi Consultancy offers a range of services to ensure your cosmetic products comply with UK regulations
Representation & Advocacy
We represent and protect your brand to the relevant UK authorities, ensuring your interests are well-defended.
Legal Guidance
Our team provides expert advice on any legal matters relating to UK Cosmetics Regulation, helping you navigate complex requirements.
Customer Complaint Management
We assist in handling any consumer complaints or product-related issues, maintaining your brand’s reputation.
Regulatory Monitoring & Compliance
We stay on top of regulatory updates and ensure your products remain fully compliant with evolving UK laws.
Managing Adverse Effects
We oversee the management and reporting of any undesirable effects your cosmetic products may cause.
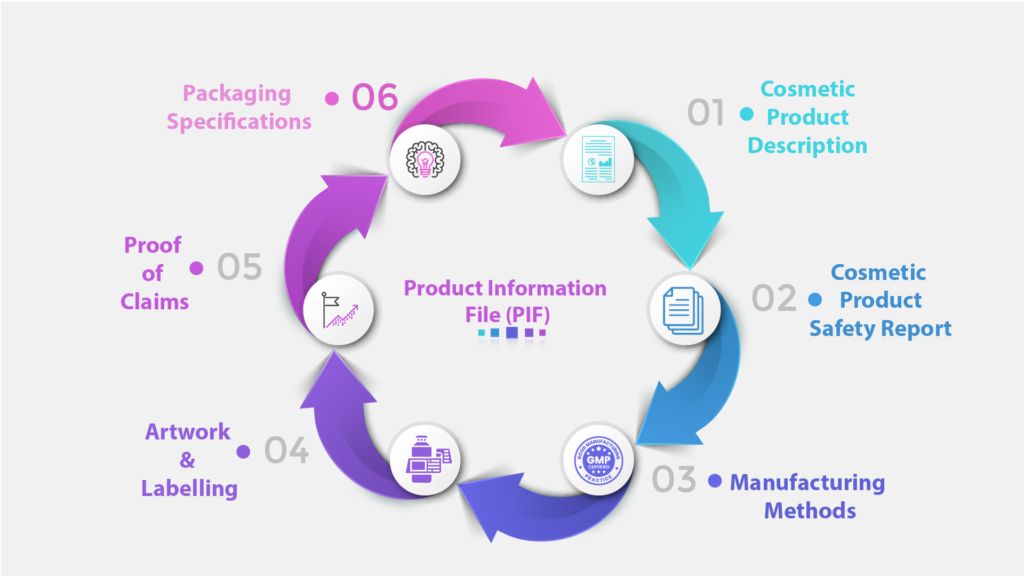
Product Information File Management
We ensure your Product Information File is always current and compliant with UK requirements.
Comprehensive Solutions for Cosmetic Compliance
Our Solutions
Partnering with a skilled and experienced UK Responsible Person simplifies the complexities of cosmetic regulations, minimising risks and ensuring seamless market access. Eomi Consultancy stays ahead of regulatory changes, ensuring your products continue to meet UK standards, keeping your business compliant, competitive, and future-ready.
PIF compilation and review
Eomi Consultancy provides comprehensive Product Information File (PIF) preparation services, ensuring cosmetic products meet UK regulatory standards. Our meticulous approach guarantees that all required documentation is complete, accurate, and fully compliant.
- Detailed compilation of product information file
- Review & Approval of product information file
- Continuous updates to reflect regulatory changes
- Ensuring all regulatory requirements are met
Product Notifications (CPNP, SCPN)
At EOMI Consultancy, we manage the entire product notification process, ensuring timely and seamless submissions to the relevant regulatory authorities.
- Preparation & Submission of Notifications
- Liaison with Regulatory Authorities
- Ongoing Compliance Management
- Quarterly Regulatory Updates
Claims for Cosmetic Products in the UK & EU
Ensuring your cosmetic products meet regulatory standards is essential for maintaining market access and consumer trust. At Eomi Consultancy, we provide expert support in crafting effective, compliant, and compelling cosmetic claims. Our team helps you navigate the complexities of regulations, ensuring your products stand out while meeting all necessary requirements.
CLAIMS SHOULD
- Be Truthful: All claims must be backed by scientific evidence and must not mislead consumers.
- Be Clear: Claims should be easy for the average consumer to understand and not ambiguous.
- Be Substantiated: Claimed benefits must be supported by appropriate testing, data, or reliable documentation.
- Be Honest: Claims must accurately represent the product’s performance and effects without exaggeration.
- Be Fair: Claims should be objective and avoid misleading comparisons with competitors or their products.
- Be Relevant: Claims should specifically relate to the product’s intended use and actual benefits
CLAIMS SHOULD NOT
- Be Misleading: Claims must not create false impressions about the product’s benefits, performance, or characteristics.
- Be Ambiguous: Claims should not be vague or open to multiple interpretations that could confuse consumers.
- Be Unsubstantiated: Claims should not be made without adequate supporting evidence.
- Be Exaggerated: Claims should not imply results beyond what can be realistically achieved.
- Be Comparative Without Justification: Claims must not unfairly disparage or misrepresent competitors unless supported by verifiable evidence.
- Be Irrelevant: Claims should not imply benefits unrelated to the product’s actual function.
Claims Substantiation
Ensuring your cosmetic products meet regulatory standards is essential for maintaining market access and consumer trust. At Eomi Consultancy, we provide expert support in crafting effective, compliant, and compelling cosmetic claims. Our team helps you navigate the complexities of regulations, ensuring your products stand out while meeting all necessary requirements.



Labelling & Artwork

A label is your product’s first impression and a key compliance factor. Navigating complex regulations can be challenging, but EOMI Consultancy ensures your cosmetic labels and artwork meet EU and UK requirements for seamless market approval.
Our expert review covers:
* Ingredient Listings & INCI Compliance
* Mandatory Labelling Information
* Warnings & Precautionary Statements
* Language & Placement Compliance
* Claims Substantiation & Artwork Accuracy
Formulation Review and INCI list preparation
At EOMI Consultancy, we understand the importance of compliance and professionalism in the cosmetic industry. Manufacturers are legally required to disclose the full list of ingredients on all cosmetic product packaging, and adhering to the International Nomenclature of Cosmetic Ingredients (INCI) is essential for global consistency.
Our expert team ensures your formulations are compliant with EU and UK regulations while providing accurately formatted INCI lists. We also offer allergen advice to help you meet labelling requirements and safeguard consumers. Whether you’re a mainstream beauty brand, a natural product formulator, or a start-up brand, our services guarantee your ingredient list is both compliant and professionally presented, helping your products stand out in today’s competitive market.

CPSR and Lab support
At EOMI Consultancy, we provide end-to-end support for Cosmetic Product Safety Reports (CPSR) and lab testing, ensuring that your products meet all safety and regulatory requirements. Our services include:
* Lab & Safety Assessor Coordination
* Test Form Completion Assistance
* Arranging Necessary Product Testing
* Test Results Analysis
* Maintaining Testing Reports
* Product Safety Recommendations as per reports
We help you navigate the process from start to finish, ensuring that every product you release is thoroughly tested, compliant, and ready for the market. Whether you’re looking to arrange testing, interpret results, or maintain proper documentation, our team is here to support you every step of the way.

Initial Regulatory Strategy and Roadmap
At EOMI Consultancy, we help define the best regulatory strategy and roadmap for your product. We assess your product’s specifics and determine the most suitable regulatory pathway for market entry. Our team ensures you understand what’s required to choose the right pathway and guides you every step of the way.
We provide an overview of the necessary actions, including classification, safety assessments, and documentation required for approval. For borderline products, we help you navigate whether they fall into regulated or unregulated categories and advise accordingly.
Our services include:
* Identifying the optimal regulatory pathway
* Outlining necessary steps for market entry
* Offering guidance on borderline product classification
* Suggesting required documentation and approvals

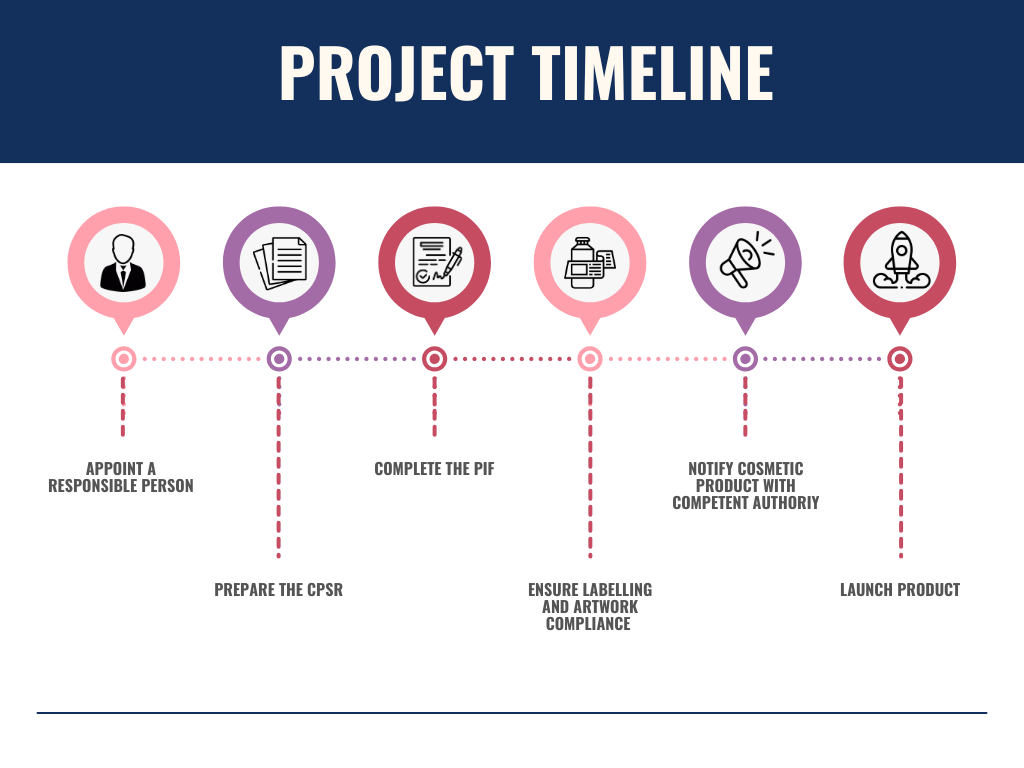
Steps to Appoint Eomi Consultancy as Your UKRP for Cosmetics
- Contact Us for a Free Consultation
Reach out to our team via phone, email, or through our contact form for an initial consultation. We will discuss your specific needs and assess your product’s compliance requirements. - Initial Assessment & Documentation Review
We will review your cosmetic product range, existing regulatory documents, and ensure you meet the necessary UK cosmetics regulations. - Sign the UK Responsible Person Agreement
Once we understand your requirements, we will provide you with a tailored contract and service agreement outlining our role as your UKRP. - Submit Required Regulatory Documentation
Provide the necessary documentation, such as your Product Information Files (PIF), labeling, and any safety assessments required for the registration. - Ongoing Compliance Management & Post-Market Surveillance
As your appointed UKRP, we will manage ongoing compliance and provide post-market surveillance services, ensuring your products stay in full regulatory compliance across the UK market. - UK Market Access
Once everything is in place, your cosmetic products will be legally able to enter the UK market, with Eomi Consultancy acting as your official UKRP.